
Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.
The atomic number of each element increases by one, reading from left to right. Summary Atomic Number Protons, Electrons and Neutrons in Caesium Caesium is a chemical element with atomic number 55 which means there are 55 protons in its nucleus. There are also at least 39 artificial isotopes created in a lab.
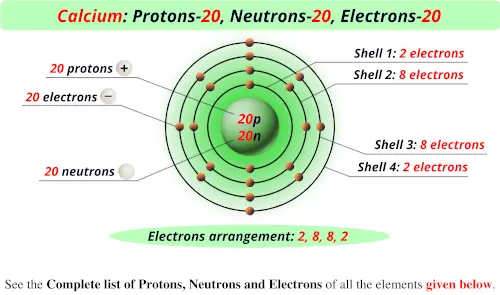
Period A horizontal row in the periodic table. Boiling point: 1,239.8 F (671 C) Number of natural isotopes (atoms of the same element with a different number of neutrons): 1. How many neutrons calcium However, the number of neutrons in the nuclei of the calcium atoms can vary. This element has 20 protons, 20 electrons, and 20 neutrons giving it an atomic mass of 40. Members of a group typically have similar properties and electron configurations in their outer shell. How many protons neutrons and electrons does CA have Calcium.

Trending Questions What are the three subatomic particles in an atom? What types of electrons s p d or f are considered those involved in the make up of the outer shells of atoms? What is the formula of Calcium acetate? How many moles of magnesium are present in 1 billion atoms of magnesium? How many atoms of phosphorus are contained in 5. Cs Caesium 55 132.905 Glossary Group A vertical column in the periodic table.


 0 kommentar(er)
0 kommentar(er)
